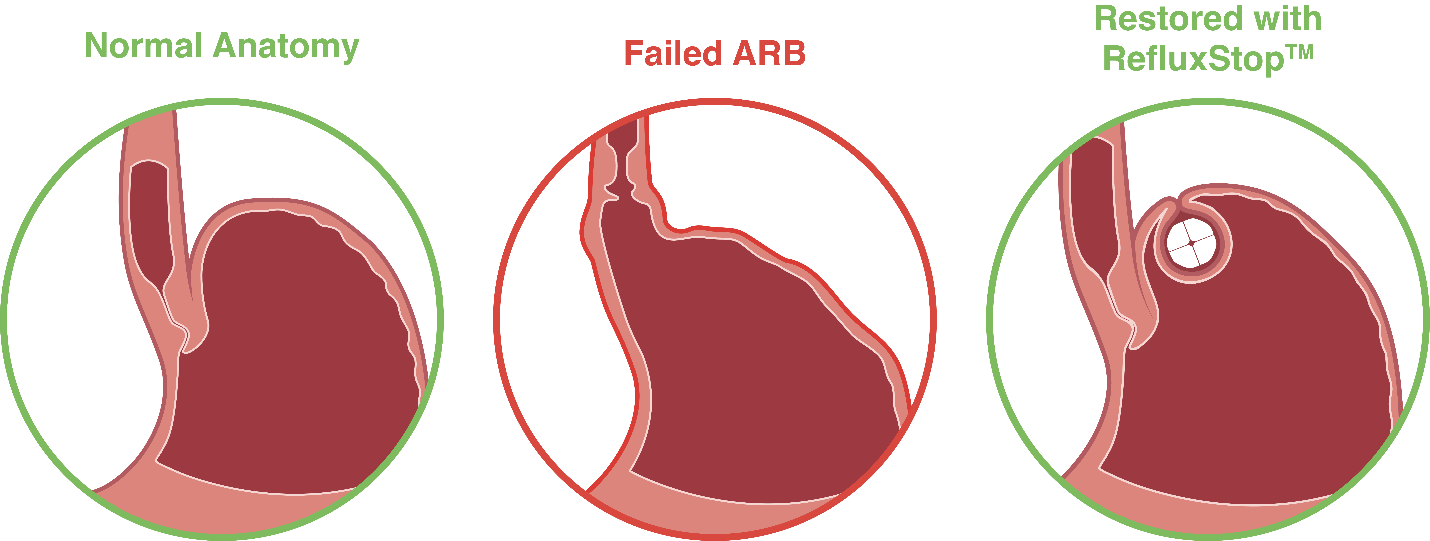
RefluxStopTM restores and maintains the position of the body’s natural Anti-Reflux Barrier (ARB) components, allowing it to function normally. Click here to read American Foregut Society’s whitepaper on the Anti-reflux Barrier.
With RefluxStopTM, the food passageway is not encircled therefore unpleasant side effects associated with other anti-reflux procedures are kept to a minimum.
If left untreated, chronic acid reflux can lead to serious damage to the esophagus such as inflammation, ulceration and tissue changes. Such tissue changes can lead to a precancerous condition known as Barrett’s esophagus1, affecting 10-20% of daily acid reflux sufferers2.
Excellent RefluxStop™ 3-year CE mark clinical trial results:
Effectiveness at 3-year follow-up*
- 100% of the patients stopped taking regular daily PPI medication
- 98% had no acid reflux, as measured by 24-hour pH monitoring in the lower esophagus at 6 months
- 96% did not have any swallowing problems
- High patient satisfaction, with only 1 patient dissatisfied with the outcome (2%)
- No device was explanted
- No device specific complications
* 3 patients withdrew from the study, none of them taking PPIs at the time of withdrawal
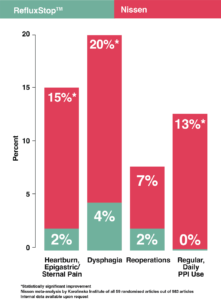
RefluxStopTM in comparison to standard of care, Nissen Fundoplication
RefluxStopTM:
- Restores the body’s natural physiology
- Can be used in conjunction with other electrical implants
- Does not impair swallowing
- Does not prohibit imaging
- Is minimally invasive
- Is well tolerated
Visit https://www.implantica.com/im-refluxstop/ to learn more
Sources & References:
- The Karolinska Institute 2020; Cancer Research UK 2014; Lagergren et al. 1999
- Modiano N, Gerson LB. Barrett’s esophagus: Incidence, etiology, pathophysiology, prevention and treatment. Ther Clin Risk Manag. 2007 Dec;3(6):1035-145.